Poisoned by Plastic: A Closer Look at HPLC Analysis of BPA and Plasticizers in Water Bottles
Q&A with Adam Moore, PhD, HPLC Applications Chemist at Hamilton Company
Introduction
In the application note titled, “Poisoned by Plastic: The Analysis of BPA and its Derivatives in Water Bottles”, Adam Moore, PhD, explores the presence of plasticizers — Bisphenol A (BPA) and BPA-derivatives — and the possibility of leaching from consumer plastic bottles. Dr. Moore addresses three hypotheses to investigate the issue. These include:
- How many BPA or similar derivatives are in the sample?
- If there are BPA or BPA derivatives in the sample, can a bottle be purged of the residue?
- Does purging of the leached material affect the structural integrity of the bottle?
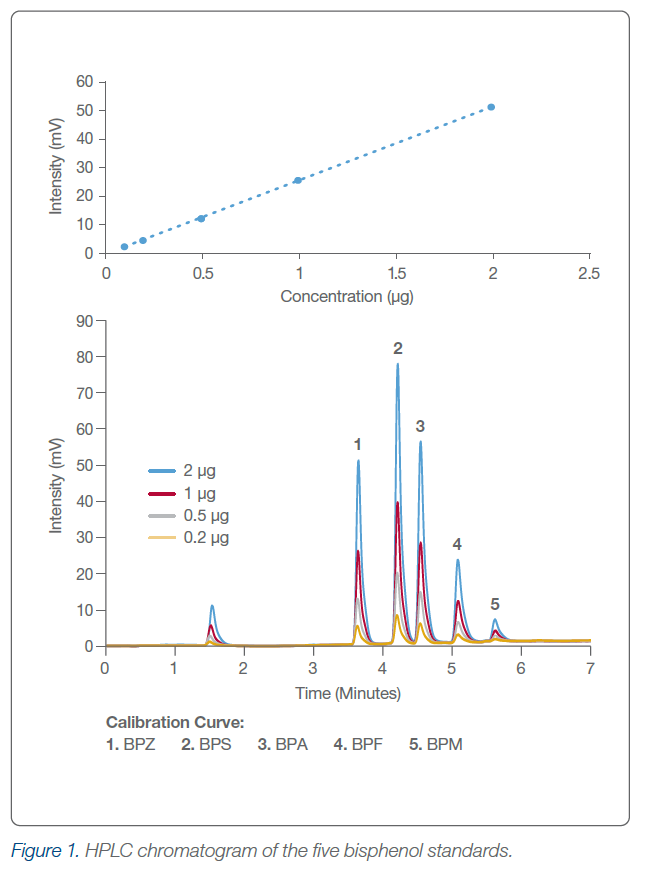
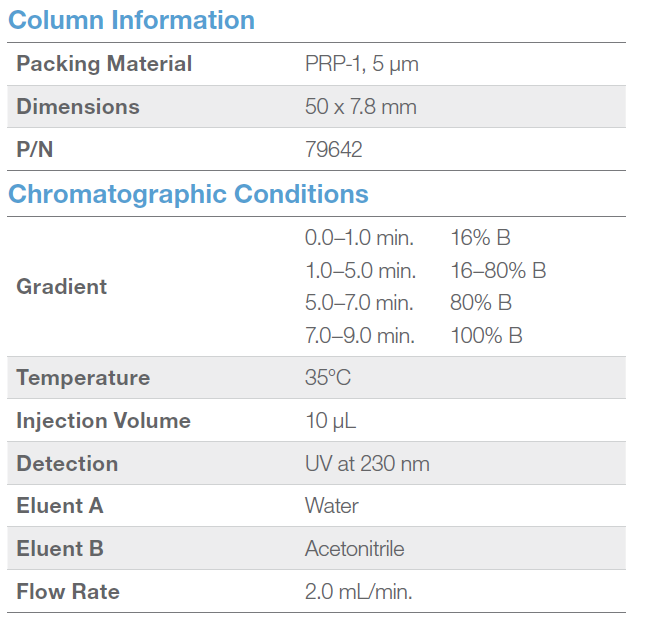
Through a set of careful experiments, Dr. Moore discovers that BPA related compounds are indeed present in plastic bottles following extraction with water or ethanol. Both the temperature of the water and the concentration of the ethanol in the extraction show linear relationships to the concentration of compounds identified. Furthermore, multiple washes with either water or ethanol resulted in stepwise decreases in detectable compounds to below the limit of detection (LOD) of the instrument. Compound analysis was performed using a Shimadzu 20A HPLC instrument with UV detection. Bisphenol derivatives were resolved using a 50 x 7.8 mm column (Hamilton) featuring a 5 µm PRP-1 particle with guard column.
Summary from the Article
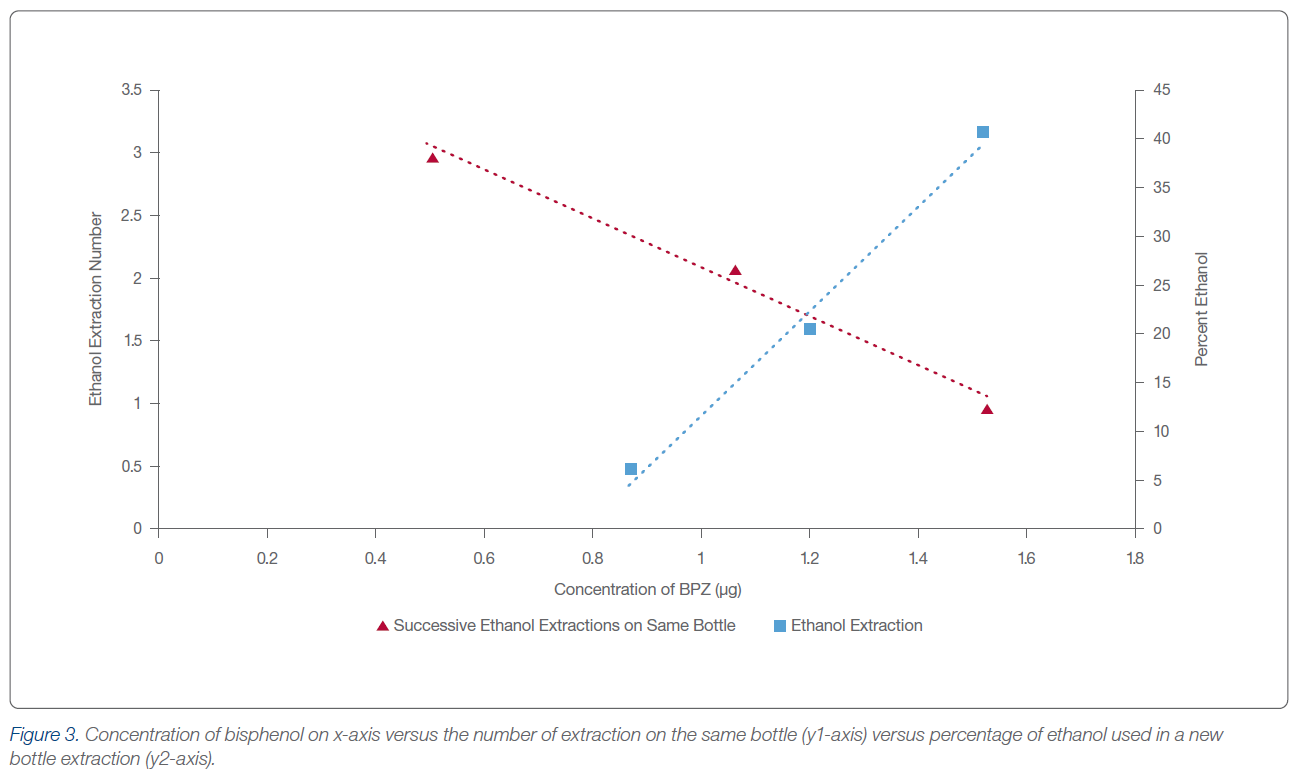
The results from this study indicate a fast and easy way to identify bisphenolic compounds through the use of liquid chromatography. The mini study indicates that new water bottles containing BPA Free labeling still contain bisphenol derivatives that can leach under exposure to alcohol or water at a temperature above ambient. To eliminate the threat of leaching toxic bisphenol, water must be kept at or below 25°C. Additionally, removal of leachable bisphenols from new polycarbonate bottles was achieved through the washing of the bottle with five repeated exposures of 40% ethanol. This produced satisfactory results that gave values below the detection limit (0.05 μg) of this study while appearing to maintain the structural integrity of the container.
Q&A
Damon Anderson (LabX): This application note is an interesting piece focused on the detection of leachable BPA related plasticizers in plastic bottles. The impact of plastics on human health and the environment is a very important and timely subject. What motivated you to explore the topic?
Adam Moore: One day I was filling up my own polycarbonate water bottle and I wanted something other than ice cold water, so I filled the bottle up with warm water and noticed a slight taste difference. This got me thinking, could this old water bottle be leaching something? The bottle said that it was BPA Free. So after doing some literature searches, I realized that BPA Free containers actually contain a derivative of BPA. Plastic bottle manufacturers had come up with a solution to the stigma associated with BPA products and still have a product that hardened in a similar or better way. The more I looked the more I found these hardeners were in lots of strange products that I wouldn’t have even thought of. I thought that this would be a good jumping off point, using a water bottle, something just about everyone has now.
DA: How did you first identify the advantages of HPLC and polymeric columns on BPA and BPA-derivative analysis?
AM: Just like other HPLC methodology related applications, an initial literature analysis was done to achieve a basic knowledge of the polarizability, solubility, pKa (if applicable), and lipophilicity based on Log P data previously published. Then we collected the physical data using neutral conditions on a variety of stationary phases to see which stationary phase performed the best before plodding forward with optimizing the methodology for analyte isolation. One thing that definitely stood out was the fact that PRP-1 stationary phase consists of a PS-DVB backbone and are of a similar make up of BPA and its derivatives. The π-π interactions between the stationary phase and the analytes provide a really good match.
DA: Can you take us through the experimental setup and briefly comment on important and interesting aspects of the results?
AM: The procedure is super simple. 20 percent of the volume of the container was used as the leachate volume. The time was just two minutes of vigorous shaking with a further hold at 30 minutes, and most of that was just so the sample could cool down enough for the SPE cartridge to perform properly. After filtration through the SPE cartridge, the sample was analyzed by the PRP-1 5 µm HPLC column. Due to the non-ionizing form of the molecules, water and acetonitrile were sufficient eluents. The really interesting part of the study were the leaching experiments using ethanol. We knew based on the solubility index of bisphenol compounds that BPA derivatives should be more leachable in ethanol than hot water, but we were really surprised as to the amount. Similarly, we were glad/intrigued to find out that there is a point where leachable material ceases to leach, providing a safe bottle.
DA: What are the strengths of HPLC analysis and the use of polymeric columns on plastics analysis, and where do you foresee these solutions being applied to public health and environmental challenges in the future?
AM: The HPLC analysis is fast, occurring in under 6 minutes. The polymeric columns are great for applications like this because of their durability, overall structural stability, and ability to be easily regenerated, if needed. The column can be used to quickly analyze multiple samples per hour. The column is so functional that it can be used in conjunction with other detection devices like mass spectrometry or amperometry, if a more sensitive detection limit is needed. As far as applications, these columns are great for environmental or toxicological applications where a great number of samples are run through the column daily.
This editorial was written by LabX and published in partnership with Hamilton
Images from “Poisoned by Plastic: The Analysis of BPA and its Derivatives in Water Bottles”, reproduced with permission.
Explore Hamilton listings on LabX
Visit the LabX Chromatography and Separations Application Page










