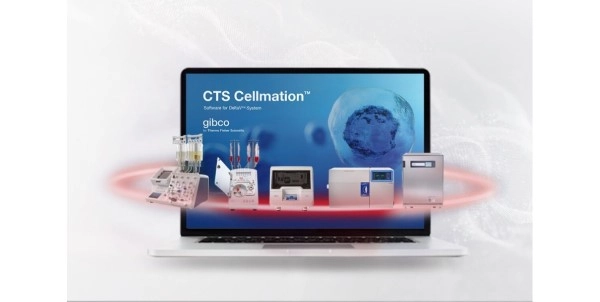
Gibco CTS Cellmation Software Digitally Connects Cell Therapy Instruments
Automating cell therapy manufacturing workflows with new, off-the-shelf software
To
optimize clinical manufacturing processes for innovators developing
breakthrough cell therapies, Thermo Fisher Scientific announced the launch of
the Gibco™ Cell Therapy Systems (CTS™) Cellmation™
Software, a first-of-its-kind automation solution
designed to connect and integrate workflows across multiple Thermo Fisher
Scientific cell therapy instruments while enabling cGMP compliance. This new
offering can help eliminate the need for costly custom software projects and
extensive validation, saving valuable time and resources during the cell
therapy manufacturing process and helping to deliver these promising curative
therapies more quickly and safely to patients. The
cell therapy manufacturing process is estimated to include upwards of 40 manual
touchpoints, increasing opportunities for errors and contamination that can
lead to failure and delay potentially life-saving treatment for those who need
it most. The CTS Cellmation Software, powered by Emerson’s DeltaV™
Distributed Control System (DCS), connects Thermo
Fisher’s cell therapy instruments within a single, user-friendly interface,
reducing the number of manual touchpoints required. By establishing an
automated workflow across multiple stages of cell therapy manufacturing, CTS
Cellmation Software helps enable traceability, repeatability and 21 CFR Part 11
compliance with secured data connectivity. “As
our biopharma partners continue to expand their cell therapy pipelines, CTS
Cellmation Software enables digital integration of unit operations, helping to
ensure reproducibility, traceability, and data security required for scaling
cGMP manufacturing processes,” said Betty Woo, vice president of cell, gene,
and advanced therapies at Thermo Fisher Scientific. “This new software offers a
fully validated, off-the-shelf automated solution to digitally connect and
control Thermo Fisher fit-for-purpose instruments, ultimately helping deliver
these potentially transformative medicines to patients more efficiently.” Thermo Fisher is committed to
streamlining manufacturing for cell therapy developers and all cell therapy
platforms currently in development will also align with and connect to this
digital, automated framework. At present, CTS Cellmation Software connects core
Thermo Fisher Scientific cell therapy instruments, including solutions from our
Gibco CTS portfolio. These instruments, along with their supporting consumables
and software products enable GMP-compliant, closed system manufacturing.