Detection of THC Metabolites in Urine Using Polymeric Reversed Phase HPLC Columns
Q&A with Adam Moore, PhD, HPLC Applications Chemist at Hamilton Company
There is a persistent need for accurate testing methods to determine THC in urine. Although an increasing number of states have legalized cannabis use, workplace policies in many of those states continue to enforce a zero-tolerance drug policy with urine testing to determine compliance. Furthermore, refined urine testing methods may aid in the determination of intoxication and safe operating levels for vehicles and other applications.
THC elimination in the body involves multiple tissues and processes. The complexity of metabolism coupled with the chemical behavior of THC makes the accurate determination of the concentration through excretion difficult. With more than 100 THC metabolites, detection of the most abundant—11-hydroxy-THC, 11-carboxy-THC glucuronide, and 11-carboxy-THC—have been the focus of most urine analysis methods to date, with carboxylate and hydroxylate metabolites accounting for 80-90% of the consumed cannabis.
To address this need for more robust and reliable THC testing methods, Adam Moore, PhD at Hamilton Company, explored the use of Hamilton PRP-C18 reversed-phase columns in detecting THC metabolites in urine. In an informative set of experiments, Dr Moore demonstrated the detection of THC metabolites at ng/nL concentrations spiked into both buffer and urine using an 8-minute LC gradient. The analysis involved column temperatures, solvents, and flow rates compatible with standard LC systems, without the need for specialized LC equipment and conditions, indicating the utility of the columns for a range of applications, including remote testing.
We caught up with Dr. Moore to ask a few questions about the study and the performance of the PRP-C-18 columns for enhanced THC metabolite analysis.
Q&A
Damon Anderson (LabX): The accurate detection of THC metabolites has been an active area of investigation for methodological and physiological reasons. Questions mainly involve the most robust biomarkers for THC metabolism and what testing platforms function best for their detection. Can you tell us what it is about the PRP-C18 chemistry that makes the columns better suited than other approaches for THC metabolite detection?
Adam Moore: In my opinion, the structures of the cannabinoid metabolites are a great fit with this stationary phase, which incorporates both the C18 functionality and the base PSDVB resin. Another advantage to using the PRP-C18 stationary phase is that it is highly durable and can be used with or without sample prep. The dilute and shoot method works well with this stationary phase. Comparing the use of this PRP-C18 method with those found in other LC-MS methods where the MS detection methods are not able to effectively ionize the acid-functionalized cannabinoids, we see that the PRP-C18 could provide an effective separation for quantification.
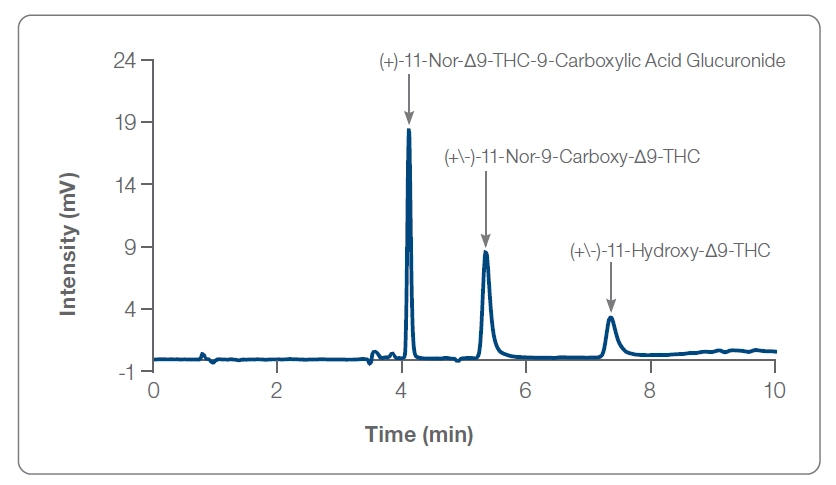
Figure 1: Three most abundant THC metabolites
DA: Have you examined the detection efficiency of the three major THC metabolites—11-hydroxy-THC, 11-carboxy-THC glucuronide, and 11-carboxy-THC—using the PRP-C18 columns? Do the sample handling conditions, the presence of urine proteins and molecules, or other parameters affect the detection profile?
AM: One of the benefits of using the PRP-C18 is that it can accommodate small proteins without clogging due to its structural pore size created during its creation. Our initial results show that the proteins in the urine sample appear to be easily removed under the ammonium acetate conditions, while the more lipophilic analytes that need to be analyzed are retained longer and allow for easy identification.
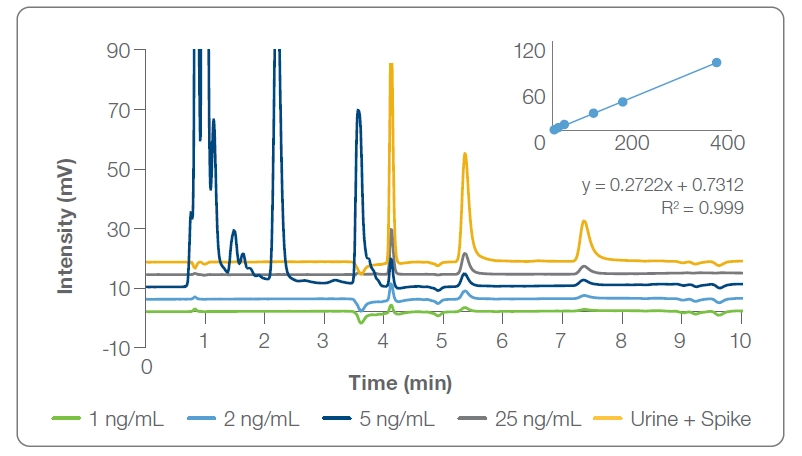
Figure 2: Range of concentrations spiked into buffer or urine (3 ng/mL)
DA: Have you measured the detection range of these columns and how this may correlate and be applicable to THC metabolite concentrations in urine samples?
AM: We have measured the detection range using the PRP-C18, and the data shows good correlation from 1 – 25 ppb. It is only a small range; however, the range shows good correlation to the positive limit of 15 ng/mL, which is assessed as the positive lower detection limit. One focus for the next study that was not addressed here is how to address the false positives and negatives, specifically where synthetic urine or adulterations are used to pass drug tests.
DA: One can envision THC metabolite detection in urine as a prime candidate for remote testing sites. Are these PRP-C18 columns fit for use on portable HPLC systems for use in the field? What chromatographic conditions of PRP-C18 LC separations are most favorable for these remote systems?
AM: Great question. These columns are a perfect fit for use in portable HPLC systems and can be easily adapted for use in the field. The durability/longevity of the resin is coupled with the sensitivity of the method. The 10 mM solution of ammonium acetate is easily prepared and used with acetonitrile. Additionally, one could imagine pre-prepped eluents to where all the analyst needs to do is add water and inject sample. The PRP-C18 makes this possible with the use of short equilibration times, universal fittings, and its rugged proprietary design. Additionally, any aromatic compounds with carboxylic acid functional groups are a great fit for this mobile phase and subsequent portable use.
DA: As a follow-up, what might be the advantages of the PRP-C18 columns for long-term use at remote locations—for instance, column life, solvent usage, time of analysis, etc.?
AM: The PRP-C18 is great for remote locations due to its durability and speed. The stationary phase allows for fast analysis with the use of higher flow rates and reduced pressure than traditional phases under similar conditions. The PRP-C18 also has the ability to provide traditional C18 retention profiles while maintaining good peak shape and resolution without showing any phase striping, thus allowing for extended lifetimes and more productivity in repeated analyses.
This editorial was published in partnership with Hamilton Company. Figures reproduced with permission.










