Complete Solutions for Microbial Testing of Cannabis / Cannabis Infused Products from Bio-Rad Laboratories
Increased legalization in the cannabis industry has translated to abundant opportunities for both emerging and established operations. Regardless of scale, cannabis production requires reliable testing and quality control to ensure products are safe. The risk for microbial contamination during cannabis processing is a serious and persistent threat -- and one that must be addressed with accurate and dependable solutions.
Bio-Rad’s iQ-Check real-time PCR kits and CFX96 Deep Well RT-PCR Detection System are powerful technologies for the detection and identification of discrete microbial species. Along with the iQ-Check Prep Automation System and RAPID’Chromogenic Media, these technologies provide fast and accurate methods for cannabis testing and quality assurance analysis -- and are ideal solutions for any scale cannabis operation.
The issue of microbial contamination in cannabis
An industry-wide lack of quality assurance guidelines underlies the wide variation in cannabis – between crops, processors, dispensaries, and even day to day operations. Although there is an increasing trend towards third party testing and microbial surveillance measures, the issue of mold and bacterial contamination is a constant threat which requires state-of-the-art solutions.
Outdoor cultivation can result in highly variable moisture levels in cannabis crops, depending on the humidity, precipitation, and soil conditions. Indoor growers must closely control humidity and vegetation density, in order to limit microbial growth as well. Measures such as anti-microbial treatments using foggers and nebulizers are often employed, as are clean conditions free from unprotected personnel, animals, and diseased vegetation. The logistics around such measures, however, can be resource intensive and are often untenable.
Handling and packaging of cannabis products also require clean procedures and close monitoring to ensure microbe-free conditions. Measures including the use of gloves, sterile wipes, sealed vacuum bags, and clean room standards are recommended, although not always adopted.
The importance of microbial detection and identification
Despite best practices, the chances for microbial growth remain high – posing a threat to consumers, patients, and processing employees, as well as reputation of the cannabis vendor.
As a world leader in PCR-based systems, Bio-Rad offers molecular and culture-based diagnostic tools validated by AOAC, AFNOR and Health Canada for the rapid detection of pathogens in cannabis products
- Bio-Rad’s iQ-Check kits - based on gene amplification and detection by the use of real-time PCR and ready-to-use reagents - are optimized for microbial detection and identification in cannabis.
- Bio-Rad’s CFX96 Deep Well real-time PCR Detection System provides a powerful platform for high-throughput microbial testing applications -- an essential complement to iQ-Check kits.
- The iQ-Check Prep PCR Automation solution is dedicated to running the complete range of iQ-Check PCR Test Kits for high-throughput microbial testing.
- RAPID’Chromogenic Media offers cost-effective labor-saving protocols to detect and enumerate microbes, quickly and thoroughly.
The iQ-Check real-time PCR technology
The iQ-Check technology is based on PCR, a powerful technique used to generate many copies of DNA. During the PCR reaction, several cycles of heating and cooling allow DNA denaturing, by heat, followed by primers binding (annealing) to the target region. DNA Polymerase is then used with these primers, along deoxynucleotide triphosphates (dNTPs), to extend the DNA, creating copies of the target DNA called amplicons.
In real-time PCR, specific probes are used to detect DNA during amplification by hybridizing to the amplicons. These probes are linked to a fluorophore which fluoresces only when hybridized to the target sequence. In the absence of target DNA, no fluorescence will be detected. As the concentration of amplicon increases with each round of amplification, fluorescence intensity also increases.
During the annealing step of the PCR cycle, the optical detector measures this fluorescence and the associated software plots the fluorescence intensity versus cycle number. This method allows a simple determination of presence or absence of the target microorganism. A synthetic DNA internal amplification control is included in the reaction mix. This control is co-amplified with the target sequence and detected by a second fluorophore. It allows for the validation of negative result and is critical to comply with quality control standards.
The iQ-Check real-time PCR solution for cannabis microbial testing
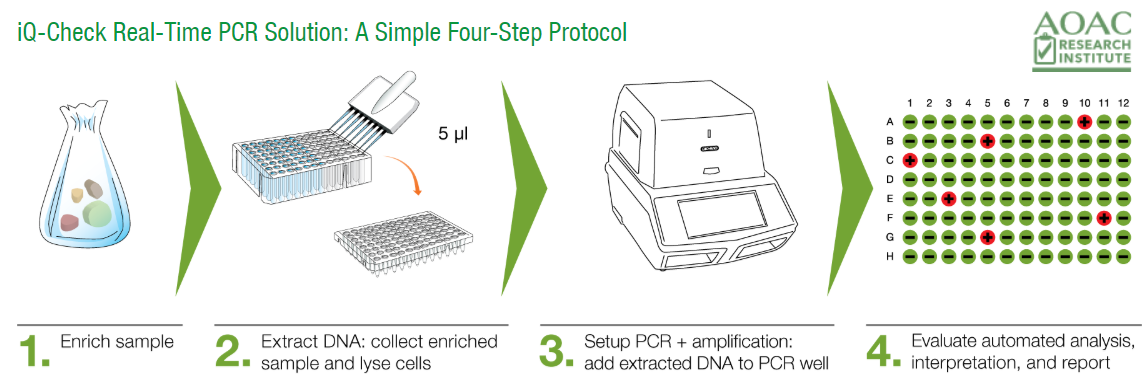
The iQ-Check RT-PCR solution has been engineered to dramatically reduce sample preparation time without sacrificing the specificity or sensitivity of the assay. The simple workflow involves 4 easy steps:
1. Sample enrichment: Sample is added to the appropriate enrichment broth and incubated overnight to allow target bacteria to resuscitate and multiply to a detectable level.
2. DNA extraction: Using a combination of lysis reagent and heat, bacterial cell membrane is disrupted in a few easy steps and DNA is extracted.
3. Real-Time PCR: The ready-to-use PCR reagents are aliquoted into the PCR plate and 5 ul of extracted DNA from each sample is added to the PCR plate. The plate is placed into the pre-programmed thermal cycler and the appropriate program is selected to begin the PCR amplification process.
4. Automated data analysis & interpretation: Data analyses and interpretation is made simple with a proprietary software that automatically analyzes real-time PCR curves and provides a qualitative output indicating presence or absence of contamination.
Currently, several iQ-Check kits are available to meet Cannabis testing regulations for the detection of Salmonella spp., E. coli O157:H7, Shiga-toxin producing E. coli (STEC), and Enterobactericeae.
iQ-Check Prep automation solution
The iQ-Check Prep automation system is a liquid handling platform designed for performing DNA extraction and plate set-up. It is optimized for use with the full range of iQ-Check microbial detection kits and the CFX96 real-time PCR system. Ease-of-use and full traceability through the CFX Manager IDE software and laboratory information management system (LIMS) make the complete iQ-Check system ideal for medium and high-throughput testing operations.
RAPID’Chromogenic Media
RAPID’Chromogenic media is available for enumeration of key bacterial contaminants in cannabis, including: RAPID’Salmonella, RAPID’Enterobacteriaceae, and RAPID’E.coli 2. The media enable easy to use protocols and easy to read chromogenic results, while allowing determination and distinction from non-pathogen species. A full suite of media kits is also available for expanded bacterial species evaluation.
Summary
Together these products offer fast, efficient, easy to use testing solutions for the range of contaminants common to cannabis production. The accuracy and reliability of these systems are designed to ensure microbial contamination is keep to a minimum and operations continue to run safely and effectively.
Although microbial contamination is an ever-present threat, Bio-Rad’s cannabis testing products offer state-of-the-art solutions for superior quality control and assurance.
This editorial was written by LabX and published in conjunction with Bio-Rad.
View Bio-Rad listings at LabX.com
Bio-Rad is a trademark of Bio-Rad Laboratories, Inc. in certain jurisdictions
Bio-Rad Laboratories, Inc. develops, manufactures, and markets a broad range of innovative products and solutions for the life science research, clinical diagnostic and food markets. Bio-Rad is a world leader in PCR testing with over 50,000 systems placed worldwide for research and diagnostic use. The company’s aim is to transfer the latest technology and methods from research to routine application, by partnering with industry to meet their needs.
Bio-Rad Laboratories is truly a global company with global solutions. We are uniquely positioned to offer first class partnership through service and support to customers worldwide. Our key product lines, iQ-Check and RAPID’Chromogenic media have undergone extensive internal and external evaluation on a variety of matrices. We look forward to partnering with you and earning your trust as an approved supplier of food safety solutions.
Bio-Rad’s real-time thermal cyclers are covered by one or more of the following U.S. patents or their foreign counterparts owned by Eppendorf AG: U.S. Patent Numbers: 6,767,512 and 7,074,367.