AQUIOS STEM System to Provide a New Solution for Stem Cell Analysis
The AQUIOS
STEM System enables complete automation to minimize hands-on time by 95 percent
INDIANAPOLIS, IN — Creating
the industry’s first stem cell analysis update in nearly 25 years, Beckman Coulter
Life Sciences—a global leader in laboratory automation and innovation—launches
an evolution of the gold standard in hematopoietic stem cell enumeration, the
AQUIOS STEM System. The advancement comes as more
than 100,000 stem cell transplantations are expected to be performed this year. “For many patients this is the
last treatment option, so we know that timely and accurate diagnostic
information is perhaps more critical than ever,” said Dr. Andreas Böhmler,
Director of Strategic Marketing for Clinical Solutions. “The success of a stem
cell transplantation largely depends on the number of viable stem cells, and we
designed the system to improve efficiency without compromising accuracy, all of
which can make a difference in patient outcomes. Stem cells can save lives, and
we’re excited we can make a difference by helping to accelerate analyzing and
enumerating viable cells.” The system runs on the AQUIOS CL
Flow Cytometer, the first true load-and-go flow cytometer that combines sample
preparation and analysis in one compact platform. Easy to use and easy to
train, the AQUIOS STEM System brings stem cell enumeration closer to where it
belongs, the patients. Full traceability is enabled by
barcoded vials to ensure a comprehensive audit trail and simplify quality
management. A single tube loader also allows for priority treatment of
emergency samples, enabling laboratory staff to handle urgent requests. The
system can reduce the need for laboratory developed tests (LDTs), fostered by a
flexible IVD assay setup, including three different validated panel options and
innovative automated algorithms, allowing labs to maintain compliance
regardless of the guidelines they follow. The first successful stem cell
transplantation took place 65 years ago. As laboratory workflows and needs
continue to evolve, adaptability is key, and the AQUIOS STEM System is the
first stem cell analysis advancement in nearly 25 years. In all test case scenarios, the
AQUIOS STEM System required substantially less hands-on time than the predicate
method. For a typical 5-day work week with 10 specimen samples per day, the
AQUIOS STEM System reduces manual workload by approximately six hours, allowing
labs to allocate resources more efficiently. In addition, the AQUIOS STEM
System reduces the overall turnaround time from sample preparation to patient
result, which is essential for a time-critical test such as CD34+ enumeration. The AQUIOS STEM System, comprised
of AQUIOS STEM Software for the AQUIOS CL Flow Cytometry System, AQUIOS
STEM-Kit Reagents, AQUIOS STEM CD34 Control Cells and Flow-Check Fluorospheres,
is an in vitro diagnostic (IVD) medical device intended to be used by
laboratory professionals for the detection of parameters in the most used
specimen types. For more information, please
visit www.beckman.com/aquios-stem. *
Compared to an alternative method ** AQUIOS
STEM System is not available in all countries. Pending submission and clearance
by the United States Food and Drug Administration; not yet available for in
vitro diagnostic use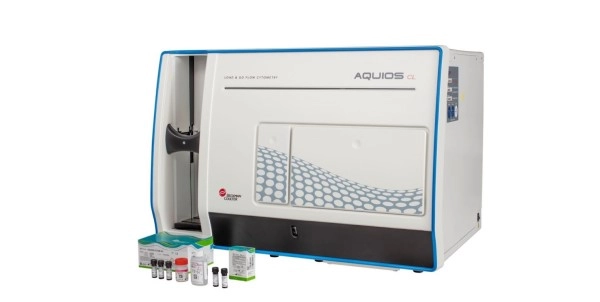 The AQUIOS STEM System enables
complete automation to minimize hands-on time by 95 percent, reduces error-prone
steps by 87.5 percent, and decreases turn-around time*. The system was designed
with leading experts in the field of clinical CD34+ enumeration, and is a
modular approach to automated and IVD-certified analysis of CD34+ hematopoietic
stem and progenitor cells.
The AQUIOS STEM System enables
complete automation to minimize hands-on time by 95 percent, reduces error-prone
steps by 87.5 percent, and decreases turn-around time*. The system was designed
with leading experts in the field of clinical CD34+ enumeration, and is a
modular approach to automated and IVD-certified analysis of CD34+ hematopoietic
stem and progenitor cells.
in the US.










