Immunoreagents and Emerging Applications in High-Resolution Imaging and Microscopy
Antibodies and immunoreagents are used in a myriad of experimental contexts
We start with a quick review of fundamentals about the synthesis, structure, and function of antibodies.
The ensuing discussion is focused on emerging applications of antibodies and immunoreagents in advanced multimodal imaging and high-resolution microscopy.
What’s an antibody?
Also termed an immunoglobulin, an antibody is a type of protein used by the immune system to target and neutralize pathogens such as viruses and bacteria. The complementary region of an antibody, Fab or paraepitope, binds to a protein epitope or antigen on the target microbe. By this antibody “tagging” mechanism, immune cells such as macrophages can home in on and destroy invaders.
Which cells produce antibodies?
Antibodies are produced as part of the humoral immune system otherwise known as adaptive immunity. Plasma cells, which are differentiated from B cells, are the source of antibody secretion.
What are the types of antibodies?
The are two main types of antibodies depending on location: a soluble form is secreted and exists freely in the blood stream while a membrane-bound form remains attached to B cells and functions as a B cell receptor. The soluble antibodies serve a surveillance function monitoring potential pathogens in the bloodstream. The B cell receptors respond to antigen stimulation by activating the B cell to produce antibodies specific to the antigen, to facilitate T helper cell activation, and to prime memory B cells for future attacks.
Fab antibody
The Fab is the variable region of an antibody that is specific to a given antigen.
Fc antibody
The Fc is the conserved region that allows an antibody to communicate with other cells of the immune system primarily through a mechanism involving a conserved glycosylation site. There are 5 different antibody isotypes depending on the structure of the glycans within the Fc region, and these isotypes connect the type of invader with the appropriate humoral response. For instance, the IgE isotype is responsible for an allergic reaction involving histamine release and mast cell degranulation.
Monoclonal versus polyclonal
Polyclonal antibodies are secreted by several types of immune cells and have Fab paraepitopes that bind unique epitopes on a given antigen. Monoclonal antibodies arise from a single source and are highly specific to a single antigen binding epitope.
What are the uses of antibodies?
Obviously, antibodies play a central role in the adaptive immune system. The ability to naturally manipulate and source subtypes of antibodies, both polyclonal and monoclonal, can be exploited for the generation of immunoreagents with a wide array of binding specificities and biotechnology uses.
Enzyme Linked Immunosorbent Assay
Also known simply as ELISA, it is a testing platform that uses antibodies to detect target molecules through specific binding and emission of light from a substrate.
- The analysis material containing an unknown amount of antigen is immobilized in a tube or microtiter plate. This can be through non-specific absorption or through capture by an immobilized antibody (sandwich ELISA).
- After washing, the detection antibody is then added – which is either conjugated to an enzyme directly or is subsequently detected using a secondary antibody-enzyme conjugate.
- After washing and addition of enzyme substrate, the color emitting product is measured using an ELISA adsorbance reader.
- Multiple dilutions and replicates can increase the accuracy and precision of quantitation.
- Increasing or decreasing the stringency of the wash steps can modulate the specificity and sensitivity of detection.
- There are several ELISA types including: Direct, Indirect, Sandwich, and Competition/Inhibition.
ELISA antibodies
Either monoclonal or polyclonal antibodies can be used in ELISA applications. Monoclonals have specificity to a single epitope which allows detection and quantitation of fine differences in antigen structure. Polyclonal antibodies can be used to capture as much antigen as possible from analysis material – and monoclonals can then be used for fine detection in a sandwich ELISA.
When performing sandwich ELISAs, the capture and detection antibodies must recognize two distinct epitopes of the target molecule, otherwise competition between sites will affect detection. When using a secondary enzyme linked antibody, it must be specific to the type of primary antibody in order for proper detection. Three main factors influence detection and the level of optimization: affinity, specificity, and avidity. View an excellent resource here.
Flow cytometry antibodies
Flow Cytometry is a widely used technique to characterize cells in solution based on detection by antibody-conjugated fluorescent molecules or fluorophores. Either polyclonal or monoclonal antibodies can be used -- the former suited for detection of low level cell surface targets in a quick and cost-effective way. Monoclonal detection can offer significantly higher levels of accuracy and specificity for use with complex antibody panels and mixed cell populations. The performance of these antibodies depends on the instrument settings, cell solution, as well as the fluorophore emission wavelength and intensity. There are extensive catalogs of antibody probes and method resources available. View an excellent resource here.
Immunoassays
There are a wide variety of “Immunological Assays” based upon the antibody target molecule detection concept. Cell-signalling assays, secondary functional assays, cytotoxicity assays, and more, all are essentially enabled by this universal technique -- that has its roots as a natural phenomenon.
Luminex high performance assays
Luminex assays are flexible and powerful bead-based multiplex assays that allow up to 50 or more user-defined target analytes to be detected simultaneously in a variety of complex backgrounds including: cell culture supernatants, serum, blood plasma, and other biofluids. Color-coded paramagnetic beads coated with analyte-specific antibodies are used in combination with biotinylated secondary antibodies for detection. Strepavidin-phycoerythrin conjugates are then used for measurement. An extensive array of antibodies and reagents, as well as Luminex instrumentation, are available. View an excellent resource here.
Antibodies and imaging
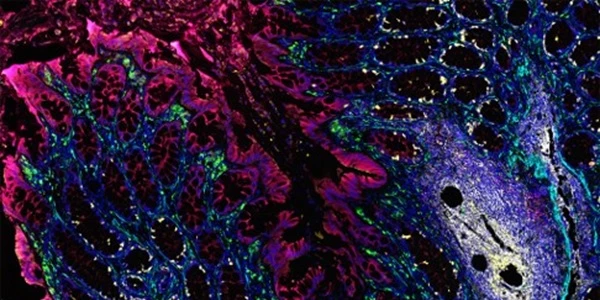
Immunofluorescence Imaging Microscopy is another very prolific application of antibodies used in biomedical research. Monoclonal and secondary antibodies coupled with fluorophores are ruotinely used to detect fine structural elements, the presence or absence of key markers, or the expression levels of specific protein in ex-vivo cell samples, sectioned tissue, live cells, and other backgrounds. The quality, specificity, and experimental context will again dictate the type of antibody, fluorophore, and optimal detection methods. View an excellent resource on these applications.
Emerging applications of antibodies in high-resolution imaging and microscopy
In addition to the proliferation of microplate imaging technologies, antibodies and immunoreagents have become invaluable in many advanced cell biological and physiological areas.
Cell imaging technologies have expanded at a rapid pace to now include: live cell imaging, high-content and high-throughput cell screening, automated microscopy, among many other applications.
Multimodal imaging and microfluidics
A combination of imaging techniques is often necessary for visualization of complex cellular processes and events. As an example, multi-modal imagers are now used to measure absorbance. fluorescence, or even FRET in a given cell imaging experiment. These in turn require changing solutions and reagents between readings, a task which contributes to complex sample workflows.
Advanced imaging platforms are making use of microfluidic devices, not only for sample preparation procedures, but for rapid, tighly controlled fluid exchange during multi-modal imaging applications.
Super-resolution imaging microscopy
The new era of super-resolution microscopy, which pushes the imaging diffraction limit to ever smaller scales, has brought with it the need to perform quality control of image processing.
Comparison of traditional diffraction limit images with super-resolution images can point out imaging defects -- which in turn can help in optimizing sample preparation procedures.
Correlative super-resolution microscopy
Correlative microscopy, the integration of two or more microscopy techniques from the same sample, is providing information across a broad range of platforms, from fluorescence spectroscopy and electron microscopy, to atomic force microscopy and beyond.
This new frontier of super-resolution microscopy depends on the integrity of experimental setup, sample prep, and data processing in order to arrive at actionable results.
A common denominator of these emerging technologies is the dependence upon the quality and performance of antibodies and immunoreagents employed in the imaging process.
View our expanding catalog of resources
Shop for antibodies, reagents, imaging, and microscopy solutions at LabX.com
Read on in the following posts:
Fluorescence Light Microscopy: Technical Insights and Applications
Progress in 3-D Imaging Microscopy and Brain Mapping
Immunoreagents and Emerging Applications in High-Resolution Imaging and Microscopy
Forging the Path of the Imaging Resolution Revolution
Ultra-High Resolution Imaging: The New Benchmark in Molecular Microscopy
Article updated June 30, 2020